Abstract
Background:
Two global, randomized, placebo (pbo)-controlled phase 3 studies of single-agent ixazomib (ixa) maintenance therapy are currently ongoing for newly diagnosed MM patients following primary therapy that included autologous stem cell transplantation (ASCT) (MM3, NCT02181413) or excluded ASCT (MM4, NCT02312258). Both trials have demonstrated statistically significant and clinically meaningful improvement in their primary endpoint of progression-free survival (PFS): for MM3, median 26.5 months (mos) ixa vs 21.3 mos pbo (hazard ratio [HR] 0.720, 95% confidence interval [CI] 0.582-0.890, P=0.002); for MM4, median 17.4 mos for ixa vs 9.4 mos for pbo (HR 0.659, 95% CI 0.542-0.801, P<0.001). Data for the key secondary endpoint, OS, have not previously been published.
Methods:
Full methods have previously been reported (Dimopoulos, Lancet 2019; Dimopoulos, J Clin Oncol 2020). Eligible patients (MM3, N=656; MM4, N=706) were randomized 3:2 to receive maintenance therapy with single agent ixa or pbo for a maximum of approximately 24 mos (26 cycles, to the nearest complete cycle) or until progressive disease or unacceptable toxicity, whichever occurred first.
Results:
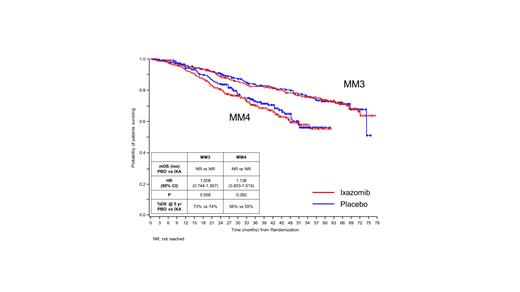
At the most recent data cut-off (MM3, 29 January 2021; MM4, 15 October 2020), with median follow up of 64 mos and 36 mos, respectively, 27% (MM3, 174/656) and 29% (MM4, 203/706) of the intention-to-treat (ITT) population had OS events. In MM3, the 5-year Kaplan-Meier estimate for OS was 74% for ixa and 73% for pbo, though the median OS had not yet been reached in either arm (HR 1.008, 95% CI 0.744-1.367, P=0.958; Figure). In MM4, the 5-year Kaplan-Meier OS estimate was 55% for ixa and 56% for pbo, though the median OS had also not yet been reached in either arm (HR 1.136, 95% CI 0.853-1.514, P=0.382; Figure). No new safety signals were identified, and the incidence of new primary malignancies in both studies was similar between ixa and pbo.
Conclusions:
These most current OS data for MM3 and MM4 have not demonstrated a statistically significant difference for the ixa or the pbo arm to date. After 64 mos of follow-up in MM3, the risk of OS does not differ between the study arms. After 36 mos of follow-up in MM4, the OS HR shows a trend that favors the pbo arm. Because interim analyses of OS may be overrepresented by deaths in patients who did not benefit from maintenance therapy, it is not known to what extent these results will be reflective of the ITT population at the time of the final analyses. As treatment options, including anti-CD38 mAb and other new mechanisms of action, for salvage therapies following progression continue to expand, OS is increasingly being confounded by subsequent therapies. Hence, demonstrating OS advantage for early line MM therapies is becoming increasingly challenging. OS data continue to be collected in these studies for later analyses.
Dimopoulos: Takeda: Honoraria; Amgen: Honoraria; Beigene: Honoraria; Janssen: Honoraria; BMS: Honoraria. Lonial: AMGEN: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Merck: Honoraria; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria. Chng: Johnson and Johnson: Honoraria, Research Funding; BMS/Celgene: Honoraria, Research Funding; Takeda: Honoraria; Abbvie: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria. Iida: Takeda: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Chugai: Research Funding; Celgene: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Glaxo SmithKlein: Research Funding; Amgen: Research Funding; Abbvie: Research Funding; Bristol-Myers Squibb: Research Funding. Mateos: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird bio: Honoraria; Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria; Oncopeptides: Honoraria. Morgan: BMS: Membership on an entity's Board of Directors or advisory committees; Jansen: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees. Kumar: Takeda: Current Employment, Current holder of stock options in a privately-held company. Suryanarayan: Takeda: Current Employment. Vorog: Takeda: Current Employment. Fergus: Takeda: Current Employment. Labotka: Takeda: Current Employment.
Use of the oral proteasome inhibitor ixazomib as maintenance treatment for multiple myeloma following stem cell transplantation or induction therapy in newly diagnosed patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal